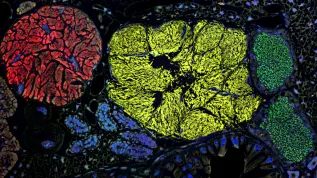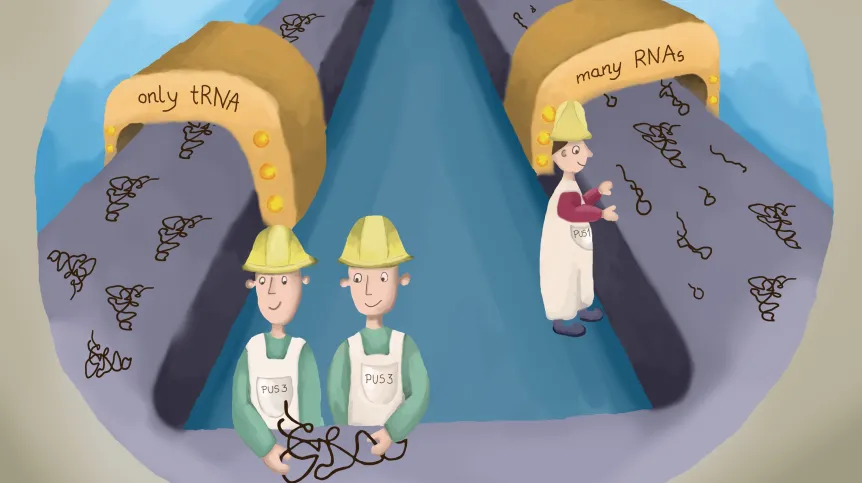
Researchers have described how the human pseudouridine synthase 3 recognises, binds, and modifies specific sites in transfer RNAs (tRNAs). Recently, pseudouridines gained particular attention, due to their utility for mRNA SARS-CoV2 vaccines.
Pseudouridylation is a crucial nucleic acid modification that affects the structure and function of almost all cellular RNA molecules, reports the Małopolska Centre of Biotechnology of the Jagiellonian University.
While the modified version of uridine was discovered more than 60 years ago, around the same time as the structure of DNA, biotechnologists still know very little about the enzymes that can introduce this modification into human RNAs. The study by scientists from the Jagiellonian University and the University of Bern was published in Molecular Cell. It paves the way to a mechanistic understanding of the unfortunate relationship between mutations and the occurrence of serious disorders.
The scientists from the Max Planck Research Group at the JU Małopolska Centre of Biotechnology determined the first cryo-electron microscopy (cryo-EM) structures of the human enzyme PUS3. The same group linked specific patient-derived mutations in PUS3 with rare human diseases.
The latest study reveals the structure of the resting human PUS3, which is +waiting+ for its target substrate, but also in different tRNA molecules. 'We looked at PUS3 from several perspectives, which allows us to draw a conclusive overall picture of this clinically highly relevant human enzyme', says Dr. Sebastian Glatt, co-author of the paper.

He explains that the obtained spectrum of structures confirms that two identical PUS3 enzyme molecules form a so-called homodimer, which is crucial for both the structural integrity of the protein and its capability to bind tRNAs. To better understand the prevalence of pseudouridylation sites in the cell, the Leidel group in Switzerland used engineered human cell lines. The authors also established a reporting system, enabling them to monitor and confirm the impacts of different clinical mutations on the activity of the enzyme. These cell culture experiments were carried out in collaboration with the JU Faculty of Biochemistry, Biophysics, and Biotechnology.
'Pseudouridine synthases were discovered decades ago, and we are excited that the advent of new techniques helps us expand our knowledge of the PUS3 homodimer complex and its enzymatic activity as well as how it achieves substrate selection', says the first author of the study, Dr Ting-Yu Lin.
Data were collected on the Titan Krios G3i, a high-end cryo-electron microscope, located at SOLARIS National Synchrotron Radiation Centre. The work at MCB JU was supported by an ERC Consolidator grant currently implemented in Dr. Glatt's group.
Ph.D. Sebastian Glatt is one of 120 newly elected EMBO members. In the area of structural biology, he conducts research on translation regulation. EMBO will officially welcome new members at the turn of November in Heidelberg, Germany. The list of all newly elected EMBO members and associate members is available here.
PAP - Science in Poland
kol/ agt/kap/
tr. RL