
Scientists from Poland and Italy have identified the structure of the protein responsible for sensing cold. This protein plays a role in soothing pain, among other things, which is why in the future their findings may contribute to the development of new medical therapies for neuropathic pain and irritable bowel syndrome.
The two-year research project, conducted using cryo-electron microscopy technology, was aided by the AlphaFold artificial intelligence algorithm, which was used to model selected protein fragments. The results of the study were published in the journal Communications Biology, as reported by the Warsaw International Institute of Molecular and Cell Biology.
Receptors located in the nerve endings of our skin are responsible for the perception of temperature and other external stimuli. Among them, proteins of the TRP (Transient Receptor Potential) family play a key role.
They form ion channels, hollow structures that enable the controlled passage of selected ions through the cell membrane. Activation of these channels leads to depolarisation, i.e. an equalisation of ion concentrations inside and outside the nerve cell, which generates a nerve impulse carrying information about the detected stimulus. The importance of this topic was evidenced by the 2021 Nobel Prize awarded to David Julius and Ardem Patapoutian for their research on temperature and touch receptors, including TRPM8 proteins.
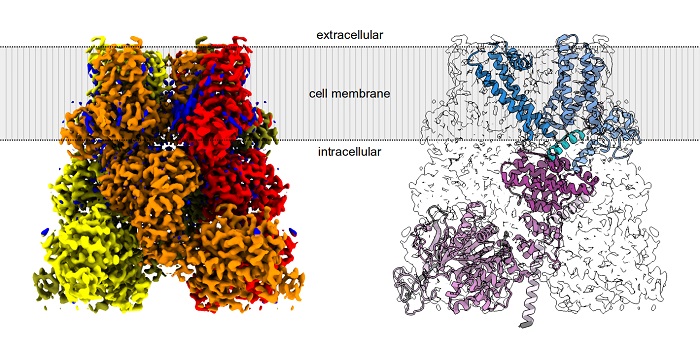
Scientists from the International Institute of Molecular and Cell Biology in Warsaw (IIMCB), led by Professor Marcin Nowotny, in collaboration with Italian researchers led by Dr. Carmine Talarico from Dompé Farmaceutici SpA, determined the structure of the human TRPM8 protein and created a model of its binding to icilin, a synthetic compound that is 200 times more potent than menthol in activating the TRPM8 ion channel.
'We know that certain chemical compounds stimulate our receptors. For example, menthol in peppermint activates TRPM8 cold receptors, hence the cooling sensation when eating mints. However, stimulation of these thermoreceptors is also associated with the perception of pain, and saturating their action through prolonged exposure to cold or menthol can reduce the sensation of pain. Therefore, a detailed understanding of thermoreceptor function is of great importance and may ultimately lead to the development of therapies for diseases related to these ion channels. These include neuropathic pain, irritable bowel syndrome, oropharyngeal dysphagia, chronic cough and hypertension', says Dr. Mariusz Czarnocki-Cieciura from the Laboratory of Protein Structure at the International Institute of Molecular and Cell Biology in Warsaw.
The researchers argue that understanding the mechanism action of a particular protein requires studying its structure, specifically how the polypeptide chain folds in space. Ion channels, however, are challenging objects of study for structural biologists. They are anchored in the biological membrane, which complicates many experiments. Due to difficulties in obtaining human TRPM8 protein, the first structural studies of this ion channel were performed relatively recently, in 2018 on avian proteins and in 2022 on murine proteins.
Structural biologists emphasise the huge importance of a method called cryo-electron microscopy (cryo-EM). This technique, developed rapidly in recent years, represents a breakthrough in the study of molecular structures, which until recently was extremely labour-intensive. Cryo-EM involves visualizing molecules using a high-resolution electron microscope at extremely low temperatures. This method has enabled the discovery of the structure of many membrane proteins, including numerous ion channels in the TRP family.
'This is the technology we used in our research', says Dr. Czarnocki-Cieciura. 'Thanks to the use of the KRIOS electron microscope at the National Synchrotron Radiation Centre SOLARIS in Kraków, we were able to visualize individual molecules of this ion channel and precisely determine its structure'.
The researchers also used the AlphaFold artificial intelligence algorithm developed by Google DeepMind. 'AI-based modelling methods have revolutionised structural biology. They enable us to predict protein structures using computers, but it should be emphasised that they do not replace costly and time-consuming experimental studies. However, they are an excellent complementary method - they help to formulate new hypotheses and support the interpretation of results, significantly speeding up certain stages of research', explains Professor Marcin Nowotny, head of the Protein Structure Laboratory at IIMCB. (PAP)
PAP - Science in Poland
lt/ bar/ kap/
tr. RL













