
Scientists have captured the high-resolution structures of one of the key proteins of photosynthesis in plants, recording a moment in the catalytic cycle. They found that water is forced out of the protein's active site through channels on its surface in a way that resembles the action of a syringe plunger.
High-resolution structures of a plant protein show how one of the key components of the photosynthetic power plant of the cell works.
The discovery, published in Nature Plants, reveals how photosynthetic proteins work at the molecular level. The researchers have shown in detail the catalytic reactions that take place in these proteins. Thanks to these reactions, plants can convert solar energy into forms they can use to grow and survive. While expanding our understanding of how plants function, these findings could also contribute to the development of new technologies related to the conversion of solar energy and improving the efficiency of plant cultivation.
The research results that shed new light on the processes occurring in plant cells are the work of scientists from the Department of Molecular Biophysics of the Faculty of Biochemistry, Biophysics and Biotechnology and the Max Planck Research Group of the Małopolska Centre of Biotechnology of the Jagiellonian University.
As the scientists explain, during photosynthesis, plant cells convert light energy into chemical energy. Photosystems have a key role in this process; they cooperate with the enzymatic protein complex - cytochrome b6f, creating a system of molecular machines that act like cellular power plants.
Professor Artur Osyczka explains that the task of cytochrome b6f is to carry out chemical transformations of the plastoquinone molecule, which acts as an electron transporter in this system. This process is necessary for generating the energy the plant needs to live. Thanks to cryoelectron microscopy, the researchers managed to capture cytochrome b6f during the catalytic cycle, which revealed the surprising position of one of the key molecules.
'The structures revealed the existence of specific channels filled with water molecules that connect the catalytic site with the protein exterior. Based on these observations we propose a mechanism that resembles the action of a plunger in a syringe', says Dr. Sebastian Glatt, quoted on the Jagiellonian University website.
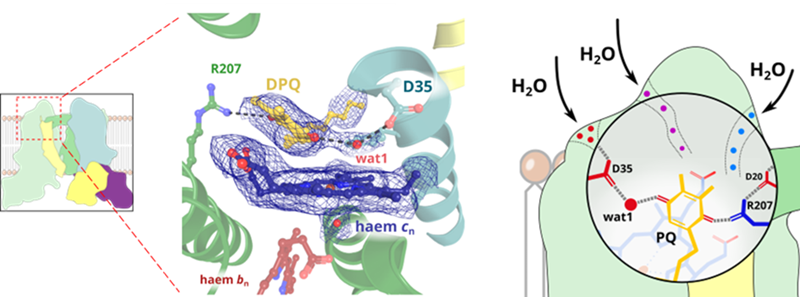
'A specific single water molecule is placed in between the quinone and most likely directly participates in the chemical reaction', says Dr. Rafał Pietras, one of the authors of the study. 'When the substrate enters the cavity, water molecules are pushed out through these channels, and after the reaction when the product leaves the site, water can flow back into the cavity. We anticipate that such more efficient quinone-water exchange mechanisms are common in similar catalytic sites', adds Professor Artur Osyczka.
All the recently described have been been obtained based on data from the Titan Krios G3i high-end cryo-electron microscope located at SOLARIS National Synchrotron Radiation Centre. The high-resolution structure described in Nature Plants represents the best-resolved structure of cytochrome b6f from higher plants ever obtained. At an overall resolution of 1.9 Å it is even possible to unambiguously identify individual water molecules within the large membrane protein complex. The structures will provide useful models for quantum mechanical calculations.
The research was financed by the Polish National Science Centre (OPUS grant awarded to A. Osyczka) and the Foundation for Polish Science (TEAM grant awarded to A. Osyczka, and TEAM TECH Core Facility grant awarded to S. Glatt). (PAP)
PAP - Science in Poland
kol/ bar/ kap/
tr. RL













