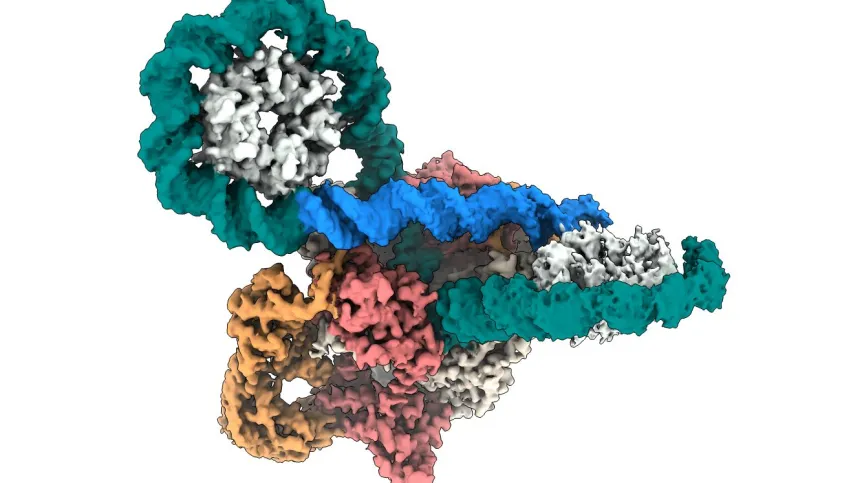
Scientists from the Jagiellonian University, alongside international collaborators, have revealed unprecedented high-resolution images of DNA gyrase, a critical enzyme for bacterial survival.
This breakthrough, using advanced cryo-electron microscopy (cryo-EM), offers new insights into the enzyme’s intricate function and opens the door for the development of more effective antibiotics, particularly against drug-resistant bacterial infections.
The research, published by the Małopolska Centre of Biotechnology (MCB) at Jagiellonian University, in collaboration with Durham University and the John Innes Centre, represents a major step in understanding a key target for antibiotic therapies: DNA gyrase, an enzyme found only in bacteria.
Unlocking the Mechanism of Bacterial Survival
DNA gyrase is crucial for bacterial life. It is responsible for supercoiling bacterial DNA, a vital process that allows bacteria to package their genetic material tightly within their cells. Without this function, bacteria would be unable to survive or replicate. The research team used cryo-EM, a cutting-edge imaging technology, to capture the enzyme in action at a resolution never achieved before, shedding light on its complex behavior at a molecular level.
As described by the researchers, DNA gyrase acts like a molecular machine, twisting and stabilizing bacterial DNA in a way that is essential for bacterial survival. The process of supercoiling resembles the action of twisting an elastic band—except that instead of unwinding, the enzyme ensures that the DNA remains tightly coiled and stabilized, ready for bacterial processes such as replication and transcription.
“The enzyme works by wrapping DNA into a figure-eight loop, then breaking and passing strands through one another before resealing them,” explained Elizabeth Michalczyk, a PhD candidate at the Małopolska Centre of Biotechnology and first author of the study. “This is a very delicate process, and if the DNA strands remain broken, it can lead to bacterial cell death.“
Targeting Gyrase: A Pathway to New Antibiotics
This vulnerability of DNA gyrase has been exploited by existing antibiotics like fluoroquinolones, which block the enzyme’s ability to reseal the broken DNA strands, ultimately causing bacterial cell death. However, bacterial resistance to these drugs has become a growing issue, complicating treatment options for various infections.
The new findings, which reveal the precise mechanism of gyrase action, could be a game-changer in the design of next-generation antibiotics. By understanding how the enzyme works at the molecular level, scientists can develop more targeted and effective drugs that bypass existing resistance mechanisms.
“By capturing gyrase in high resolution, we can now see how it interacts with DNA and the exact order of events that occur during supercoiling,” said Professor Jonathan Heddle from Durham University, a co-author of the study. “This detailed understanding could inform the design of novel inhibitors that more precisely target gyrase and circumvent resistance.”
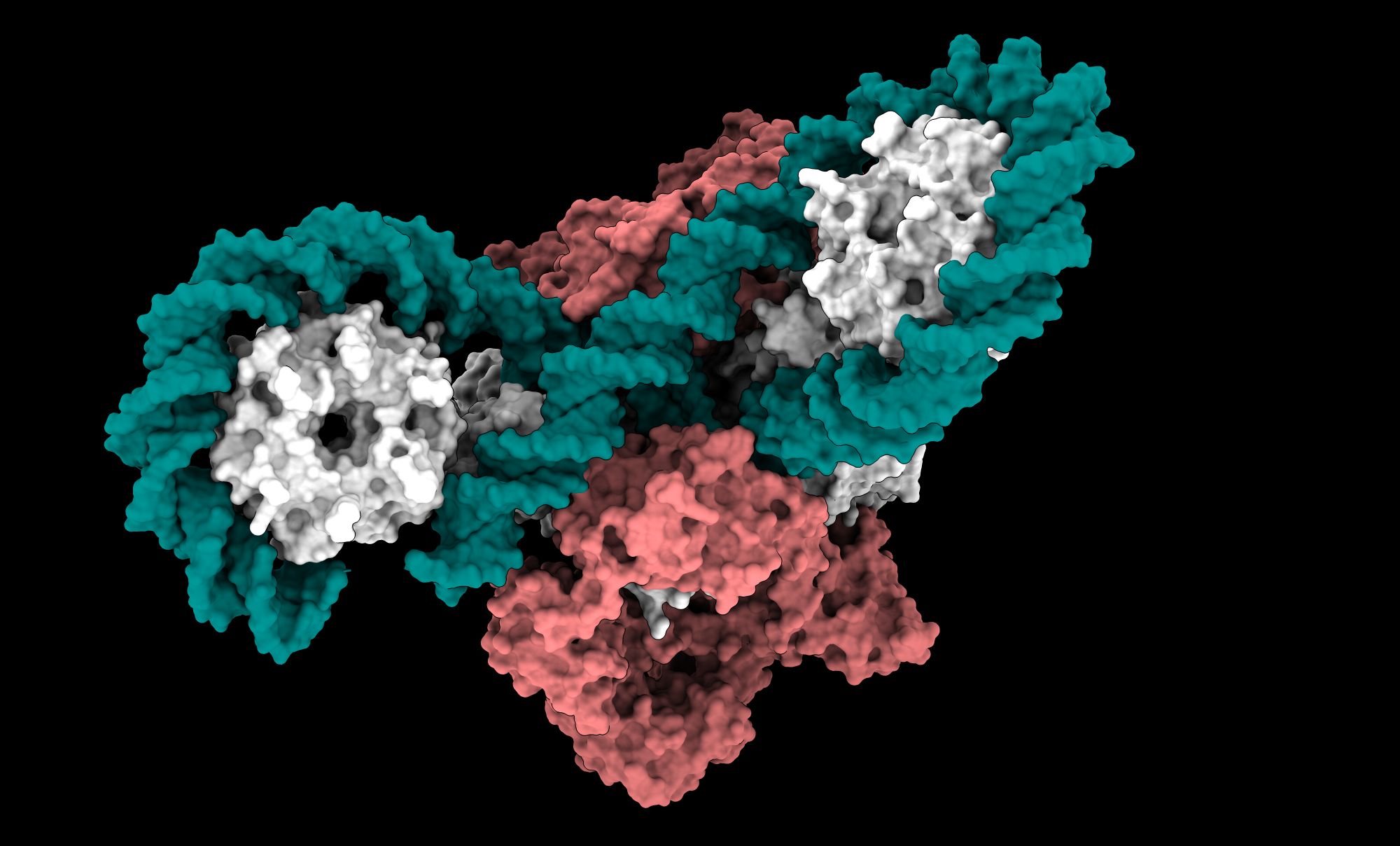
Cryo-EM: A Revolutionary Technique
Cryo-electron microscopy (cryo-EM) was instrumental in this discovery. Unlike traditional electron microscopy, which requires samples to be coated in metal, cryo-EM allows researchers to observe biological molecules in their native, frozen state. This technique provides extraordinarily detailed images at near-atomic resolution, revealing the structural intricacies of proteins and enzymes like DNA gyrase.
The cryo-EM data were collected using the Titan Krios G3i electron microscope at the SOLARIS National Synchrotron Radiation Centre, a state-of-the-art facility in Poland. The breakthrough results provide a comprehensive view of the gyrase enzyme as a highly coordinated, multi-part system, where each component works in a precise sequence to facilitate DNA supercoiling.
A Step Toward Precision Medicine
The scientists plan to continue investigating gyrase, taking additional “snapshots” of the enzyme at various stages of its action to create a molecular ‘movie’ of its function. This detailed understanding of gyrase’s mechanism could lead to the development of antibiotics that are more precise, efficient, and capable of overcoming the growing challenge of bacterial resistance.
“This research brings us a step closer to designing more effective antibiotics that act specifically on bacterial enzymes like gyrase, minimizing the risk of resistance,” said Dr. Chakraborty, a senior biotechnologist involved in the study. “In the long term, this could lead to a new era of targeted treatments for bacterial infections, improving patient outcomes and combating antibiotic resistance.”
Research Support and Acknowledgements
The research was supported by a grant from the Polish National Science Centre under the OPUS-20 programme. The team extends special thanks to the SOLARIS National Synchrotron Radiation Centre for providing the cryo-EM facilities and to the international collaborators who helped make this research possible.
PAP - Science in Poland
kol/ agt/ kap/
tr. RL













