
Researchers at the Jagiellonian University have developed several technologies for the market of batteries and home energy storage. They will allow manufacturers to become independent from foreign suppliers of expensive and rare raw materials: cobalt, nickel, lithium and graphite.
According to the Centre for Technology Transfer CITTRU at the Jagiellonian University, the use of one of the new technologies will help eliminate the risk of combustion of cells, which is one of the main risks associated with the use of lithium-ion batteries. Another technology will enable the production of alternative anodes, for example from starch, and another will allow to create environmentally friendly cathodes.
The creators of the solutions say that their methods meet the requirements of green chemistry. The production of some of them leaves no carbon footprint at all, and battery prototypes have comparable or better parameters than those already available on the market. They can be used in the energy storage and battery industry, including batteries for electric vehicles.
The researchers behind the technologies are from the Technology of Materials and Nanomaterials Research Group at the JU Faculty of Chemistry; the team leader is Professor Marcin Molenda. For over a dozen years, they have been conducting research on the development of ecological consumer energy: the production of new types of energy storage, the adjustment of parameters during loads and operation, and the search for ways of safe disposal or reuse of materials.
The research has led to the conclusion that it is possible to introduce systemic changes in the process of production of high-voltage batteries. According to Professor Marcin Molenda, by basing the process on green chemistry principles, it is possible to mass-produce more sustainable batteries and become independent from rare, expensive and polluting resources which are currently used in the production of energy storage devices.
STARCH ECO-ANODES
One of the technologies developed by Professor Marcin Molenda's team is CAG - a completely new method of producing anode materials based on carbogel.
Carbogel is obtained from starch, which is a fully renewable source. The starch itself is extracted from rice, potatoes and corn. It is then gelatinised with water, which means that it is still a green chemistry process. The next step is controlled pyrolysis with the combustion of the emitted gases.
According to the solution authors, CAG produces batteries without using graphite, either natural or synthetic, without compromising battery performance. Importantly, the method has a zero carbon footprint at the anode level and is based on a secure raw material supply chain. The CAG anode material can be integrated into any class of existing cathodes in lithium-ion cells. Tests have confirmed the high durability of CAG anodes at the level of over 1500 discharge/charge cycles.
'The developed carbogel can be used to produce green lithium-ion cells with a reduced carbon footprint. It also has the benefit of being made of widely available materials and, consequently, independence from foreign suppliers of graphite. CAG exhibits similar energy density to batteries with natural graphite, but it has the advantage allowing for a higher power output,’ says Professor Marcin Molenda.
ECO-CATHODES WITHOUT HARMFUL ELEMENTS
The second technology is LKMNO, a proprietary solution that enables the production of cobalt-free high-voltage cathodes with five times less nickel and two times less lithium. They are also produced in a green chemistry process.
The production has relatively low energy consumption and, importantly, does not generate any solid or liquid waste. All process gases can be converted into carbon dioxide, nitrogen and steam.
LKMNO cathodes can be easily paired with various types of anodes (including CAG) and electrolytes used in modern lithium-ion cells. This technology is suitable for the production of dedicated high power and high capacity cells, such as those used in EV batteries.
'The cost of producing a LKMNO cathode is twice as low as producing a cutting edge NMC cathode, which contains nickel, manganese and cobalt,’ says Professor Molenda. 'Additionally, our cathode uses two times less lithium than NMC cathodes while making full use of it. Other modern lithium-ion cells only make use of about 50% of their lithium's potential output, and lithium is quite expensive. So that’s purely a waste. In contrast, lithium in LKMNO cathodes is 100% efficient.’
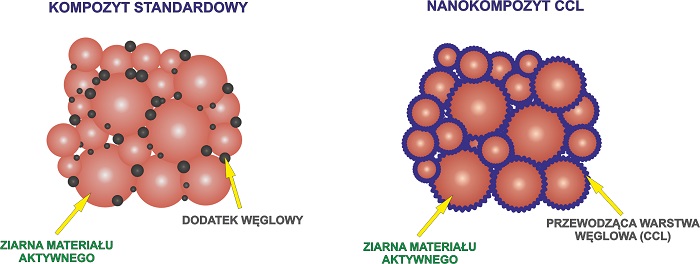
BATTERIES PROTECTED AGAINST SPONTANEOUS COMBUSTION
Chemists from the Jagiellonian University have developed one more technology - CCL (Carbon Conductive Layer). It precisely covers the active materials contained in the energy storage devices with a thin carbon coating measuring just a few nanometers. By adjusting the coating thickness, it is possible to influence such parameters of the battery as discharge time or load limit. The coating is so effective that no additional carbon material needs to be used, so the battery can have a higher energy density. However, the main advantage of the invention is its very high safety level. It completely eliminates the risk of spontaneous combustion.
'Carbon material is added to batteries to ensure sufficient conductivity. Previous technologies, however, don’t allow for precise arrangement of carbon particles between those of active materials. As a result, batteries contain a large amount of carbon, which leads to lower energy density. Because batteries are subject to changes in temperature, there is an increased risk of spontaneous combustion when the carbon material is not evenly distribute,’ says Professor Molenda.
'CCL eliminates that risk, because the particles of active materials are tightly covered, making them effectively separated from one another. In a battery like that, even if there is a short circuit, the process of discharge will happen much more slowly, negating the risk of ignition,’ he adds.
Like other technologies developed at the Jagiellonian University, CCL is also produced in a green chemistry process, and no waste is generated during production. It can be used in lithium-ion cells, and prototype tests have shown a high battery life of up to 3,000 cycles.
A STABLE SUPPLY CHAIN IS ESSENTIAL
Scientists say that over the next seven years, the global demand for electricity from various types of batteries and energy storage devices will increase at least a few, and in some industries even several times. This is primarily due to the amendment of climate regulations, although the growing market of electric vehicles and the expanding network of photovoltaic installations are also significant.
Due to the problems with the transmission of energy from prosumers to electrical networks and settling payments, the demand is growing rapidly for local energy storage facilities capable of meeting the needs of individual households and workplaces.
For example, in 2018, the demand for energy from lithium-ion batteries was estimated at 0.184 TWh per year. The latest predictions approximate that number to rise to 4.7 TWh by 2030, meaning it will increase 25 times. According to the Polish Alternative Fuels Association, by 2030 there will be 10 times more electric cars in Poland alone, despite the fact that the Polish market is growing at a slower pace than the EU average.
'Somebody will have to produce such a great number of batteries, emitting a lot of harmful substances into the environment and using a lot of rare and, consequently, expensive resources, such as cobalt, nickel and lithium, but also graphite needed for anodes,' the researchers say.
'In the face of such great and rapid development of this market, the world must already deal with two difficult challenges. The first one is the production of batteries according to rules and regulations that enforce the use of methods that are not detrimental to the planet. The second is the limited availability of resources: rare and expensive materials as well as graphite,’ says Professor Molenda. 'These elements can be mined in only a few places, and in amounts that are insufficient for the global demand. As a result, the energy storage industry is increasingly dependent on countries with access to these resources. Growing demand also leads to an increase in prices, which further aggravates economic problems. Not changing the status quo will mean a drastic increase in energy costs and a more polluted environment.’
Therefore, following the principles of green chemistry and eliminating rare, expensive and harmful elements will soon become a necessity. Even the performance and efficiency of the batteries will become a secondary issue; all this to reduce production prices, maintain a large scale, ensure a stable supply chain and meet safety requirements.
CONQUER THE MARKET
The inventions developed by the Jagiellonian University Technology of Materials and Nanomaterials Research Group are patent protected. The Centre for Technology Transfer CITTRU is working on finding a way to implement their solutions into production processes. Business partners have already been found.
'These battery technologies have an extreme potential for implementation. It is important that they are introduced by an entity with appropriate experience and scope of activity,' says Dr Gabriela Konopka-Cupiał, director of the Centre for Technology Transfer CITTRU. 'One of the options we are considering is working with an existing company seeking to improve its products while simultaneously lowering costs. We can also see a way in which a producer decides to introduce a completely new generation of batteries that will be safer, greener, cheaper and based on secure supply chains. We have several advantages that will make us strong competitors in the field,’ she says. (PAP)
PAP - Science in Poland, Katarzyna Czechowicz
kap/ bar/
tr. RL













