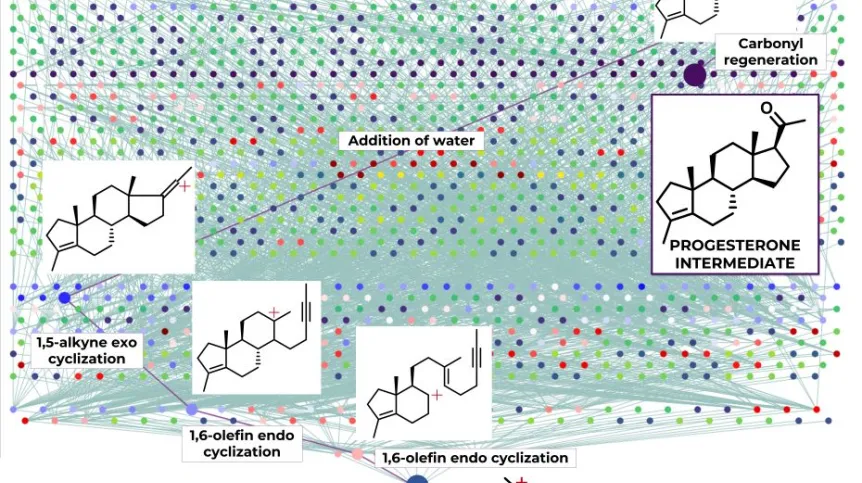
Artificial intelligence has cracked some of the most complex chemical reactions - carbocation rearrangements - and predicted their products. This is thanks to the HopCat algorithm, an integral part of the AllChemy software created and developed by Professor Bartosz Grzybowski's group.
'These rearrangements often significantly change the three-dimensional structure of the molecule, in such a non-obvious and intricate way that predicting the final results poses a challenge even to experienced chemists,’ the researchers wrote in their publication on computational prediction of complex cationic rearrangement outcomes inNature.
Within a few minutes, the algorithm can propose expected rearrangements products, present networks of possible steps leading to specific structures and calculate the probability of obtaining them.
The reliability of the results obtained from the AllChemy software and HopCat algorithm was verified by an international team of scientists from the Institute of Organic Chemistry of the Polish Academy of Sciences, the Faculty of Chemistry of the Jagiellonian University, University of Illinois at Urbana-Champaign and University of Basel, under the supervision of Professor Grzybowski's company - AllChemy.
Previous achievements in the field of artificial intelligence and neural network introduction into modern organic chemistry have revolutionized the attitude to the synthesis of complex organic molecules, like potential drugs and natural compounds.
However, the researchers say that the methods developed so far are based on already available methods and knowledge developed by humans. It is therefore necessary to distinguish AI activities from computer methods. Computer methods typically operate on full, literature-described reactions and are unable to develop novel approaches based on chemistry knowledge and reaction mechanisms.
Meanwhile, as the researchers describe, artificial intelligence 'understands' the basics of chemistry and reaction mechanisms. Based on a detailed database of physicochemical rules, quantum mechanics and kinetic calculations, it can predict the chemical structure of potential products.
AI has solved some of the most complex reactions - carbocation rearrangements. And it predicted their products. As the authors emphasise, such rearrangements can change the three-dimensional structure of a molecule in such an intricate way that it is a challenge even for experienced chemists.
The research part at the Faculty of Chemistry of the Jagiellonian University, supervised by Dr. Sebastian Baś, concerned the verification of the correctness of the software prediction with experimentally observed results from the rearrangement of carbocations obtained from natural terpenes such as linalool (present in tea) and fenchol (an ingredient of perfume).
The algorithm was not only able to explain the product distribution and provide proper mechanistic networks for observed rearrangements but also correctly predicted the temperature effect on the product's percentage content.
In addition, the AllChemy algorithm identified some additional products not reported in prior studies and enabled analytical data verification obtained from dedicated software in order to eliminate incorrectly assigned structures of the detected compounds, which was also confirmed experimentally.
The paper published in Nature was written by: Tomasz Klucznik, Leonidas-Dimitrios Syntrivanis, Dr. Sebastian Baś, Barbara Mikulak-Klucznik, Martyna Moskal, Sara Szymkuć, Professor Jacek Młynarski, Louis Gadina, Dr. Wiktor Beker, Martin D. Burke, Konrad Tiefenbacher and Professor Bartosz A. Grzybowski.
The researchers tested the algorithm with three sets of experiments. Their results confirmed that computers could not only manipulate known types of reactions, but would also help chemists rationalise and discover new, mechanically complex transformations.
Find out more on the AllChemy software website.
PAP - Science in Poland
kol/ bar/ kap/
tr. RL













