Scientists have determined how the toxic protein machinery is activated in Barth syndrome, a genetic disease that affects boys. Problems with the heart, brain and muscles cause most patients to die in early childhood. Now, the work of a 40-person group of researchers with the participation of a Polish scientist brings hope for designing a drug.
Boys with Barth syndrome suffer from serious problems with the heart, skeletal muscles, they do not grow properly and have neurological problems. Girls can only be asymptomatic carriers. The disease occurs once in 400,000. birth and develops mainly in the first decade of life. Characteristic features of children's appearance include deep-set eyes, prominent cheeks, high forehead or protruding ears. The disease also causes visual, spatial and mathematical difficulties. The prognosis of patients is very poor. Although the first case was described in 1983 by the team of Dutch scientist Peter Barth, so far no effective drug has been found. Therapy is limited to treating symptoms.
The Nicolaus Copernicus University reports that there is hope for a drug. An international research group with the participation of Dr. Karolina Mikulska-Rumińska from the Faculty of Physics, Astronomy and Applied Computer Science of the Nicolaus Copernicus University discovered the mechanism responsible for the occurrence and development of the disease. Scientists have selected a chemical compound that can be introduced into clinical tests for patients with Barth syndrome.
A group of almost 40 researchers decided to look at the issue in a more extensive and comprehensive way. Starting from biochemical and biophysical experiments, through computer modelling, and finishing with tests on animals and trials on samples from biopsies from patients with Barth syndrome, Dr. Mikulska-Rumińska has been in charge of explaining molecular mechanisms behind the disease, applying computer modelling.
'In Barth syndrome, we deal with mutation of chromosome X, and more precisely in TAZ gene, which codes taffazin, a protein which takes part in metabolism of lipids, specifically cardiolipin', the biophysicist says. She adds that cardiolipins are very important - they are found in mitochondria, which are our 'energy production plants' that power many processes in our organism.
'Once we deal with a mutation in taffazin, cardiolipins are not created, and instead monolysocardiolipins are accumulated, which are cardiolipins without one acylic bond. Then, a huge problem occurs as cardiolipins impact the functions of a hundred of other mitochondrial proteins', says Dr. Mikulska-Rumińska. She adds that cardiolipins are created from monolysocardiolipins, mainly due to taffazin. In Barth syndrome, monolysocardiolipins accumulate and a deficit of cardiolipins occurs at the same time.
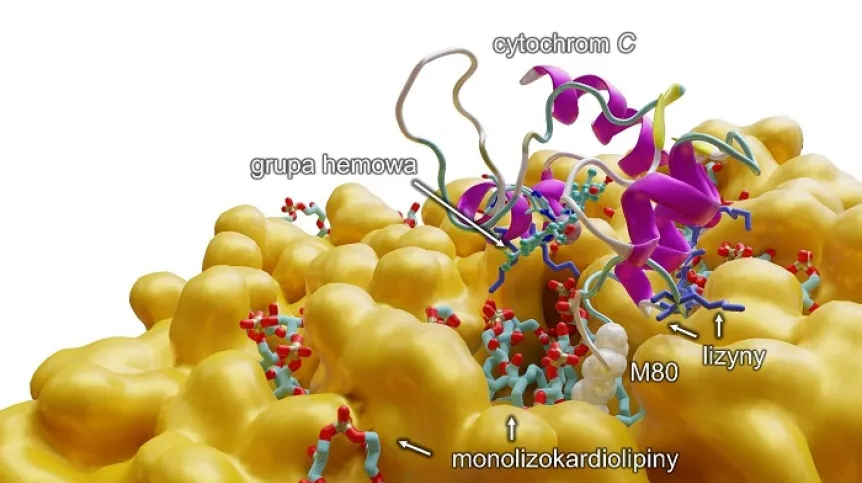
'We have managed to establish that monolysocardiolipins, which are a poorer version of cardiolipins, combine with cytochrome c. It is a very important protein, responsible, among other things, for cell respiration. It also takes part in the programmed death of the cell. We have discovered that, by connecting with cytochrome c, a monolysocardiolipin changes its function and (...) the complex begins to work as a machinery creating +toxic phospholipids+, which start to negatively impact muscles, the heart, and the brain. We have therefore proved that this complex - monolysocardiolipins with cytochrome c - is the basis and the beginning of Barth syndrome,’ says Dr. Mikulska-Rumińska.
The team has also identified a specific chemical compound that blocks the complex of cytochrome c with monolysocardiolipins. Dr. Mikulska-Rumińska used a computer model to show how the process runs. According to the researcher, this allowed scientists to better understand the molecular foundations of the studied model, which is indispensable for effective treatment.
The paper presenting the research results 'Anomalous peroxidase activity of cytochrome c is the primary pathogenic target in Barth syndrome' was published in Nature Metabolism.
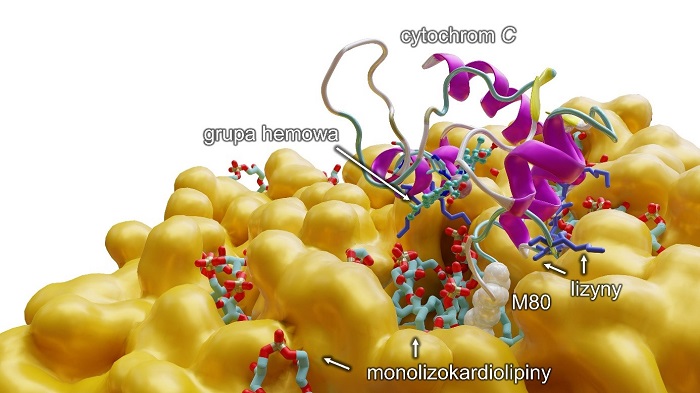
Description of the illustration: Computer modelling which shows on a molecular level the reaction of a monolysocardiolipin with cytochrome c, which results in breaking the bond between iron from the hem group and metionin 80 (M80) and the transition of cytochrome to pentacoordiation form, activating at the same time an abnormal function of the protein, which is the capability of peroxidation of polyunsaturated fatty acids (PUFA). In addition (in blue) there are lysines, amino acids with positive charge, which allow the cytochrome C to join the lipid membrane (in orange).
PAP - Science in Poland
kol/ bar/ kap/
tr. RL













