Quantum chemists from the Wrocław University of Science and Technology have described the principles of a completely new class of chemical reactions. It was discovered during research on the formation of DNA building blocks from compounds present in the environment. In these reactions, chemical processes activated by UV radiation and the so-called chemistry of weak chalcogen interactions meet for the first time.
Mikołaj Janicki, PhD, and Rafał Szabla, PhD, from the Faculty of Chemistry described their results in the prestigious journal „Angewandte Chemie International Edition”.
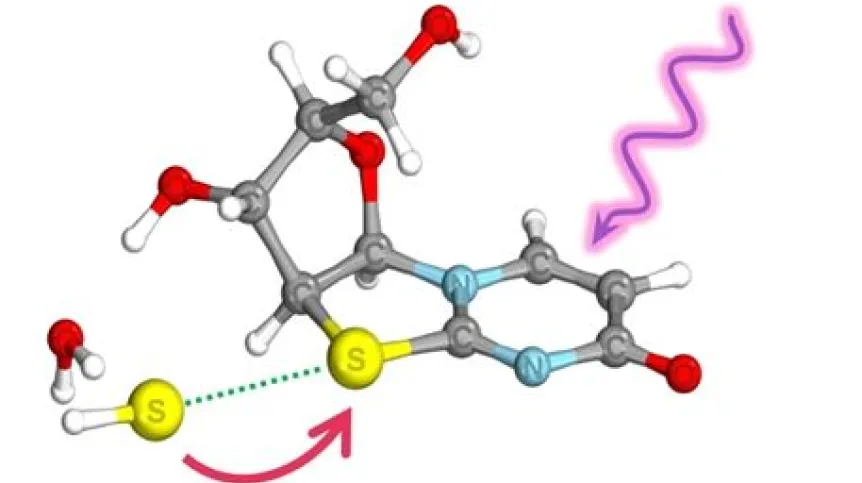
The researchers studied the reaction described by Professor John Sutherland in 2020. The Cambridge team - in collaboration with the Polish duo - showed then how to obtain deoxyribonucleotide derivatives (the building blocks of DNA molecules) from compounds available in the environment under the influence of light. British researchers knew that this reaction worked, but they did not yet know why - it could not be explained with previously known types of photoredox reactions. And it is this reaction that Polish researchers have now described.
'Until now, it was not known that photocatalysts existed that used chalcogen bonds. In this reaction, two previously separate ideas are combined', comments Rafał Szabla.
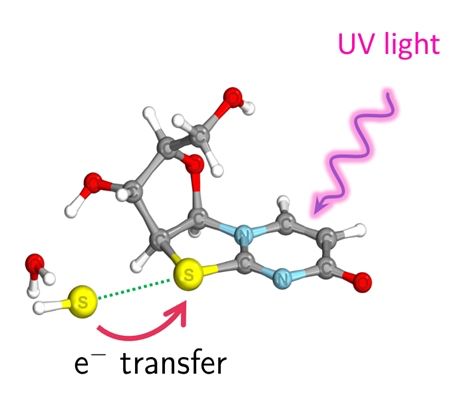
Mikołaj Janicki clarifies: 'We show that in nature, under the influence of UV, ultrafast electron transfers occur in specific complexes between photosensitive compounds and inorganic compounds. This leads to a reduction in the oxidation state and the formation of products - in this case, monomers that are part of DNA molecules', he says.
The reaction in question occurs due to the presence of hydrosulfides in the reaction mixture. Their presence gives rise to weak chemical interactions between sulphur atoms, so-called chalcogen bonds, which allow for ultrafast electron transfer in the presence of UV light, reducing the oxidation state of the organic compound.
According to Szabla, it is now worth considering whether the newly described type of reaction can be used in organic synthesis. 'It is quite possible that reactions of this type can play an important role in the synthesis of new molecules and organic compounds. While photoredox chemistry is used to synthesize complex chemical compounds, chalcogen interactions open up a range of new photochemical possibilities', he says.
Mikołaj Janicki adds: 'There are also grounds for looking for such reactions in atmospheric chemistry. There, interactions occur between sulphur atoms, but no one has studied what happens to such systems when they are exposed to UV radiation'.
'In some corners of chemistry you will no longer find new types of chemical reactions, but it seems that there is still much to discover in photochemistry', says Rafał Szabla.
Transforming energy from light into useful energy stored in chemical compounds is an important issue today. A thorough understanding of processes at the molecular level may have practical significance for designing new reaction pathways.
The research conducted by the researchers uses quantum chemistry methods. These computational methods allow us to simulate electronic structure based on the basic principles of quantum physics.
PAP - Science in Poland, Ludwika Tomala
lt/ agt/













