You did not stand here - this could be the title of the microscopic image published in Science Advanced, obtained in laboratories in Kraków, which surprised experienced biochemists. This could be a breakthrough in understanding how the 'tiny power plants' in plant cells function.
Professor Artur Osyczka from the Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University told PAP about the surprising mechanism that prompted biologists to come up with a new theory about the processes taking place inside proteins.
UNPRECEDENTED SCIENTIFIC OBSERVATION
If scientists can figure out the 'tiny power plants' of organisms, then in the very, very distant future, perhaps their successors will be able to imitate nature's ideas of and create high-efficiency molecular engines. For now, however, basic science must lay the foundation for such plans.
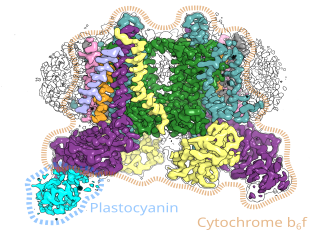
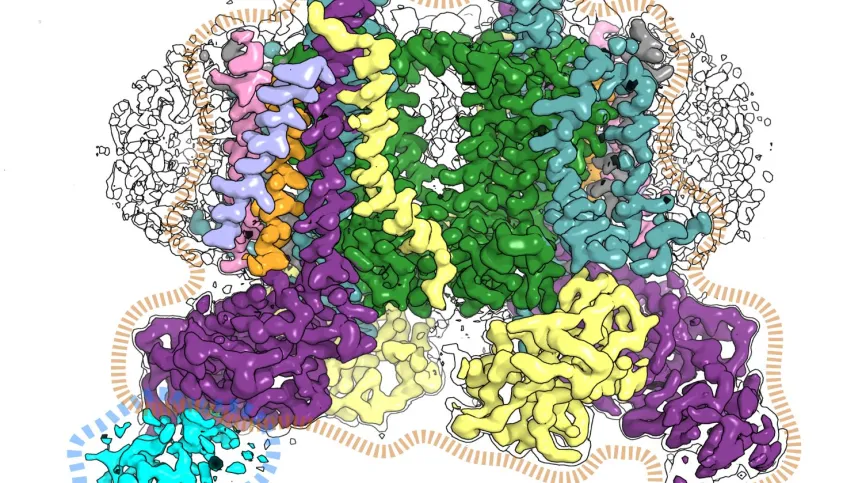
Researchers use more and more improved methods to spy on nature and find surprising patterns. In this case, they concern the systems of particles in the plant cell that convert light energy into chemical energy in the process of photosynthesis.
Living organisms use enzymes to convert energy. These are catalysts, systems direct chemical reactions so that processes such as photosynthesis can take place. Scientists from the Jagiellonian University isolated one of these proteins (cytochrome b6f) and described its cryo-EM structures.
Professor Osyczka, co-leader of the project along with Dr. Sebastian Glatt from the Max Planck group of the Małopolska Centre of Biotechnology of the Jagiellonian University, said: “A very high resolution image revealed interesting details. We saw substrates, substances needed for the enzyme to work, arranged in an unusual way. It looked as if they were waiting in line to undergo a chemical reaction. The image also suggests that they enter the protein one way and leave another - like passengers at the airport check-in.”
The obtained protein structure is equivalent to the highest quality freeze frame images. The structure presents a selected fragment of space-time, but does not directly show the movement of particles. Therefore, the substrate motion hypothesis has yet to be confirmed.
First author of the paper, Dr. Marcin Sarewicz, said: “Our discovery provides new perspective on understanding the mechanism of regulation of photosynthesis at the level of cytochrome b6f with participation of TSP9 protein.
“The structure shows that the TSP9 protein binds at a specific site to cytochrome b6f, and this protein, although known as a regulatory agent, has not been previously considered as a potential partner of cytochrome b6f.”
According to Dr. Glatt, this is so far the best-resolved structure of cytochrome b6f from higher plants. Due to its high resolution, it will provide useful models for quantum mechanical calculations aimed at further unravelling physical and chemical basis of key photosynthetic reactions catalysed by cytochrome b6f.
APPLICANTS QUEUING TO THE WINDOW
Scientists are trying to understand the enzyme's energy efficiency.
Professor Osyczka said: “Perhaps the system works like the queue system. Imagine dealing with matters in an office: there is a window with a clerk (this window is the catalytic centre of our enzyme) who is supposed to handle the applicant's case. There are people waiting in line for their cases to be handled (substrates). Until now, we thought that there was only one entrance to the enzyme's catalytic centre. We believed that the substrate, i.e. our applicant, after being served, must withdraw and leave exactly the same way it came in. Now we have reason to believe that is not the case.”
The mutual arrangement of the substrate molecules suggests the existence of a channel that enables their unidirectional flow. Interestingly, both the entrance and exit of this channel are located in separate regions of the protein. During the flow, a catalytic reaction takes place - the 'applicant is serviced'. If further research confirms the observations of scientists from Kraków, it would be a discovery of a completely new way of interaction between the protein and the substrate.
DIFFERENT ENTRY, DIFFERENT EXIT - LIKE AT THE AIRPORT
According to the hypothesis of the scientists from Kraków, 'applicants' move like travellers going to check-in counters at the airport - they enter through one entrance and leave through another. The Titan Krios G3i electron microscope at the National Synchrotron Radiation Centre SOLARIS showed a sequence of substrates suggesting that they may move in one direction inside the protein.
Osyczka said: “This makes sense, especially if the aisle for people standing in line to the counter is very narrow (as is the case with the protein channel): providing an exit on the other side could improve the process of serving applicants by ensuring that leaving applicant does not block the access of the next one. For the protein system, this would mean an increased efficiency.”
The concept of biochemists and structural biologists has yet to be confirmed. The scientists are studying both the mechanisms of energy conversion in plant cells and the mitochondria, which are the equivalent of 'cellular power plants' in animals and humans. Human and animal enzyme systems also work with great efficiency, except their energy source is not light, but chemicals.
In the case of animals, the structures of the protein studied by scientists are known, but these structures have not yet 'revealed' the substrate.
The source paper is available here.
The project was funded by the Foundation for Polish Science (TEAM grant for Professor Artur Osyczka, TEAM TECH Core Facility grant for Dr. S. Glatt).
PAP - Science in Poland, Karolina Duszczyk
kol/ agt/ kap/
tr. RL













