Polish researchers in "Cell" target the unknown SelO protein

Proteins that belong to the SelO family are found in humans, bacteria and plants, but their role has not been known. According to an American-Polish team of scientists, these proteins play a role in defending the cells against reactive oxygen species. The results have been published in the prestigious journal "Cell".
"One of the goals set by scientists is to find out what every gene is for" - says Prof. Krzysztof Pawłowski from the Warsaw University of Life Sciences (WULS-SGGW). There is still a lot of work to be done. It is estimated that the human genome contains about 20,000 protein-coding genes. "We know almost nothing about a quarter, maybe a third of these genes" - says Prof. Pawlowski.
The professor jokes that most biologists "only like the songs they know". "Biologists like to study genes that have already been studied" - he says. He adds that any new gene research project is risky. "For example, it is necessary to invent new tools to understand a new gene. It is also more difficult to obtain a grant for researching something that is not in the encyclopaedias yet" - he says.
The scientist - with an American-Polish team led by Prof. Vincent Tagliabracci from UT Southwestern, Dallas (Texas) - decided to take a risk and study a completely unknown gene. The results of their research have been published in the prestigious journal "Cell". Publications signed by Polish scientists are extremely rare in that journal.
In order to avoid wasting time on researching insignificant genes, before they start to study a new gene, scientists select those that have a good chance of being important. "A few years ago, I identified a family of genes that have not changed much since the common ancestors of humans, fungi, plants and bacteria" - the scientist says. This may mean that SelO proteins are responsible for "ancient" signalling pathways, similar in these distant groups of organisms. Another member of the team, Prof. Małgorzata Łobocka from the Institute of Biochemistry and Biophysics PAS and WULS-SGGW adds that the fact that a gene is present in both bacteria and humans means that it has existed since the times of organisms that were the common ancestors of bacteria and people, that is, it must have existed for billions of years.
What`s more, this gene is needed for the production of a rather unusual protein - SelO (selenoprotein-O). "The production of this protein is very energy-consuming. It contains the 21st proteinogenic amino acid - selenocysteine. In humans, there are only 25 proteins with this amino acid, is it`s about a per mille of all human proteins" - says Prof. Róża Kucharczyk from the Institute of Biochemistry and Biophysics PAS. The more reason to check why nature has been investing in the production of this expensive protein for so long.
Prof. Pawłowski`s bioinformatic analyses show that SelO protein should resemble the structure of kinase. Kinases are a very important group of enzyme proteins, key for transmitting signals in cells. They use ATP, the energy carrier in the cell. ATP (adenosine triphosphate) consists of adenine, sugar ribose and triphosphate. The kinases detach one of the phosphate residues from ATP and attach it to various proteins. And this changes the activity, functioning or location of these proteins in the cell.
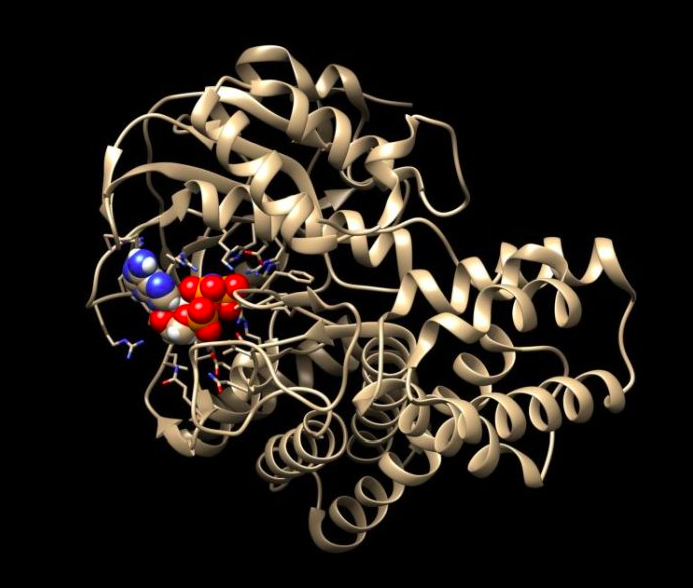
Atomic structure of SelO with bound ATP. Credit: Sreelatha et al., Cell, 175: 809 (2018). Source: Krzysztof Pawłowski
But SelO is not a kinase. It belongs to the pseudokinase group, the evolutionary "cousins" of kinases, which are not active as enzymes. In humans, they constitute 10 percent of all kinases, but their role has been unclear.
Polish-Americans research showed how this mysterious pseudokinase family works. It turns out that these proteins are active and also use ATP, but in a non-standard way. They carry out so-called AMPylation. They take two phosphate residues from ATP, but they do not consume them. They need the remainder of the compound - adenosine monophosphate (AMP). SelO attaches AMP to other proteins. It is a so far poorly understood method of changing the function of other proteins.
If you compare ATP to a cone with three scoops of ice cream, phosphate residues would be ice cream scoops, and the rest of the compound would be a cone. Kinases like one flavour of ice cream and don`t care about the cone. Meanwhile, SelO proteins get rid of two scoops of ice cream and are interested in a cone with one scoop.
The experiments show that SelO in both humans and baker`s yeast is located in the mitochondria. Yeast cells get ATP through glycolysis and mitochondrial respiration. A yeast cell needs SelO when it obtains ATP through respiration breathing. "Research on baking yeast as a research model allowed us to discover that this protein is important in the cell`s response to oxidative stress" - explains Prof. Róża Kucharczyk. In an interview with PAP she explains how researchers search for functions of unknown proteins. They use genetic engineering to remove the gene coding a given protein from a yeast cell and look for changes in the functioning of the cell. Yeast is grown on various media and subjected to various stresses. And then researchers check for differences between a wild strain and the strain lacking the tested gene. For example, it turned out that the yeast without SelO performed worse than the unmodified strain when treated with hydrogen peroxide. It can therefore be deduced that removing the gene compromises the mechanisms of defence against reactive oxygen species. These mechanisms, very complex in eukaryotic cells, are extremely important for cell homeostasis, because their proper functioning determines its life or death.
Prof. Małgorzata Łobocka`s research on bacteria led to similar conclusions. "We know that SelO is an important protein for cells. The important proteins are the targets of drugs - for example, antibacterial, antineoplastic drugs. If an insignificant protein is damaged, the cell can cope. But if we damage the protein that is important to protect the bacterium we damage against, for example, reactive oxygen species, we deprive it of weapons it uses to fight the host organism" - explains Prof. Łobocka.
She adds that bacterial and human SelO proteins are slightly different. The researcher hopes that one day we will be able to construct a compound that will impede the action of the bacterial protein, but not the human protein. "This is a beautiful beginning of research on this gene. We want to investigate how this gene affects bacterial infectivity" - the researcher concludes.
PAP - Science in Poland, Ludwika Tomala
lt/ agt/ kap/
tr. RL
Przed dodaniem komentarza prosimy o zapoznanie z Regulaminem forum serwisu Nauka w Polsce.