A whole world of possibilities opens up to scientists if they know how to lock chemical compounds in tiny cages in a controlled manner, and then open these capsules, explains chemist Dr. Wojciech Drożdż.
A team of researchers from Poland in Angewandte Chemie summarizes the possibilities offered by enclosing chemical compounds in organic and organometallic cages deposited on various types of surfaces. This is a way to build structured functional nanomaterials.
Chemists have been designing chemical structures to act as cages for some time. Under certain conditions, molecules of a selected compound - 'guests' - may spontaneously enter these cages - 'hosts'. And then, at the most desired moment, by changing the conditions (e.g. the presence of light or a change in acidity), the capsules can be opened, releasing the previously captured compounds. This allows scientists to perform many previously unthinkable tasks.
'The synthesis of compounds alone is not enough for us. We try to ensure that the compounds we develop have a chance for real applications and serve a purpose,’ says Dr. Drożdż from Adam Mickiewicz University, the first author of the publication told PAP.
CINDERELLA IN THE WORLD OF MOLECULES
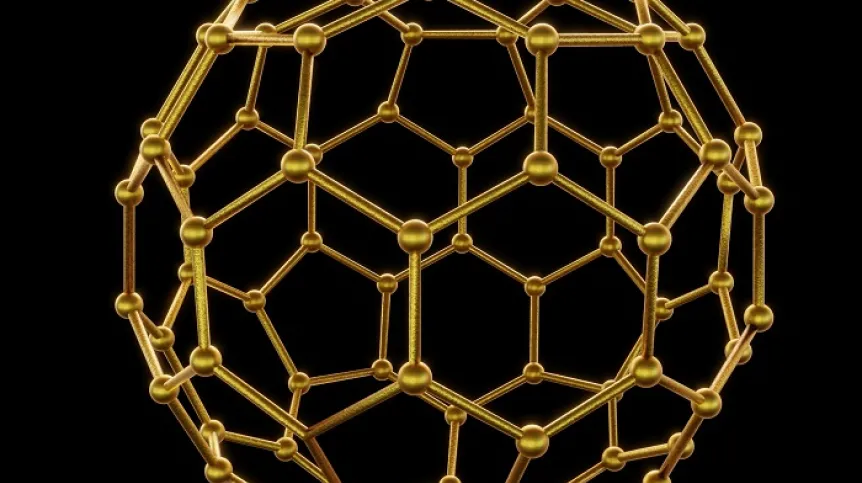
For example, caging can be used to segregate and transport chemical compounds. The group led by Professor Artur Stefankiewicz from Adam Mickiewicz University - one of the leaders of the publication in Angewandte Chemie - constructed cages for catching fullerenes. Fullerene is built of carbon atoms arranged in the shape of a football. C70 fullerenes, consisting of 70 carbon atoms, and smaller C60 fullerenes, were caught in cages developed in Poznań. In a mixture of these two types of fullerenes, C70s were more likely to pair with hosts. This example shows that cages can be a way to separate particles of slightly different sizes, shapes or properties.
The cages can also be used as components of sensor that detect selected chemical compounds.
CHEMICAL FUSE
Another application of cages is to stabilize easily reacting materials. This meant that it will be possible to use a cage to confine a compound that would otherwise quickly or violently react with the environment. It will make it possible to control the moment when the guest's reaction with the environment begins (the reaction will be delayed).
The same may apply to the isolation of catalysts, i.e. compounds that enable reactions between other compounds. The release of such catalysts will trigger (or significantly accelerate) the desired reaction - at a specific moment.
WHERE WILL THE CAGES BE?
What will the entire cage systems look like? A tempting idea is to deposit these nanocapsules on various surfaces. This would result in the development of intelligent structured nanomaterials.
'Dynamic cages can be deposited on various types of surfaces to obtain materials with properties that are better or completely different than the source materials,’ says Dr. Drożdż.
CAGES ON THREADS
Cages can also be attached to one-dimensional materials - polymers, nanotubes or nanofibers - to change their properties, e.g. conductivity. One can also use cages to connect polymer 'threads' to each other, resulting in gel-like structures. When the cage breaks down, the structure of the material will change.
CAGES ON NETS
Nanocages can also be attached to 2D materials consisting of a single layer of atoms. This method has already been used to prepare nanostructured graphene with interesting catalytic properties.
CAGES IN HOLES
There are also ideas for placing cages inside three-dimensional structures - solids - for example, between crystal nodes or in cavities in the material. The resulting material could be useful, for example, in the transport of protons or electrons.
WHAT ARE THE CHALLENGES?
The challenge chemists dealing with this area of knowledge face is to give the obtained cage systems better and better properties (e.g. stability or selectivity), so that they become of interest beyond the academic community. We also want to look for solutions that will reduce the costs of synthesis and make it more environmentally friendly,’ says Dr. Drożdż.
'This field has great potential in creating new materials that - we hope - will improve many processes we deal with every day, or give us completely new, previously unknown possibilities,’ he adds.
PAP - Science in Poland, Ludwika Tomala
lt/ zan/ kap/
tr. RL













