
A team from Białystok has an innovative idea for electrode materials used in the construction of supercapacitors. These devices are to include carbon 'nano-onions', i.e. multilayer fullerenes.
Supercapacitors - just like batteries - are used to store energy (electric charge) and act as electrochemical power sources. However, unlike batteries, they can be charged and discharged very quickly, and this process is reversible - up to a million charging cycles of such a device are possible.
DISADVANTAGES OF BATTERIES
Lithium-ion batteries seem to be irreplaceable for now, but they have a lot of disadvantages: energy is stored in chemical form, their production requires rare earth metals that are increasingly difficult to obtain, charging them takes quite a long time and a limited number of charging cycles are possible (which increases the costs of maintenance). Additionally, the chemicals they are made of may pose a safety and environmental threat.
That is why there are such hopes for supercapacitors based on organic. Although there already are supercapacitors that can be charged thousands of times faster than batteries, they are still not capable of storing enough energy. Existing lithium-ion batteries can store 20 times more energy than supercapacitors.
So for now, batteries and supercapacitors complement each other. For example, in hybrid cars and electric vehicles, batteries provide a large amount of energy to keep the vehicle running for as long as possible. And supercapacitors provide the power to move the vehicle or brake suddenly, while regenerating the device.
Scientists are therefore looking for materials with better parameters - which will both accumulate a lot of energy and charge quickly. For now, carbon materials - for example nanotubes - are often used in supercapacitor electrodes, but the search continues for materials that will perform even better.
A BALL IN A BALL IN A BALL IN A BALL
'A single fullerene is graphene rolled into the shape of a ball,’ Professor Marta Płońska-Brzezińska from the Department of Organic Chemistry, Medical University of Białystok, tells PAP - Science in Poland. In carbon nano-onions, smaller fullerenes are trapped inside increasingly larger ones - forming something like layers of an onion (or a matryoshka). A carbon nano-onion contains an average of 10 of these increasingly smaller carbon balls (Figure 1).
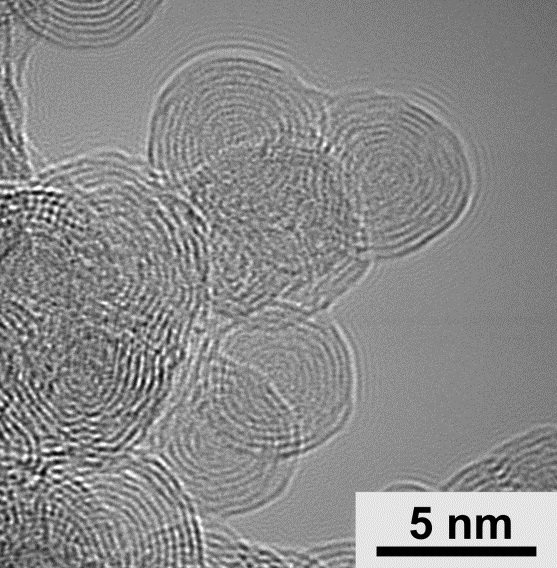
Carbon nano-onions are extremely durable, resistant to high temperatures, and can store a high electrical charge. Professor Płońska-Brzezińska explains that 'carbon balls' offer much better prospects than flat carbon materials - for example when it comes to their doping (replacing atoms of certain elements with others). In this case, it would be replacing some carbon atoms with boron, nitrogen or phosphorus atoms. The resulting heterogeneous structures would accumulate electric charge more effectively than graphene or nanotubes. That is why her team proposed using 'nano-onions' to build supercapacitors.
The method for producing nano-onions is already known, the researcher says. We know that they can be produced, for example, from diamond nanoparticles processed in appropriate conditions. 'Contrary to appearances, nanodiamonds are not expensive at all. And they are commercially easily available,’ she adds.
NANO-ONONION AS A STUFING INGREDIENT
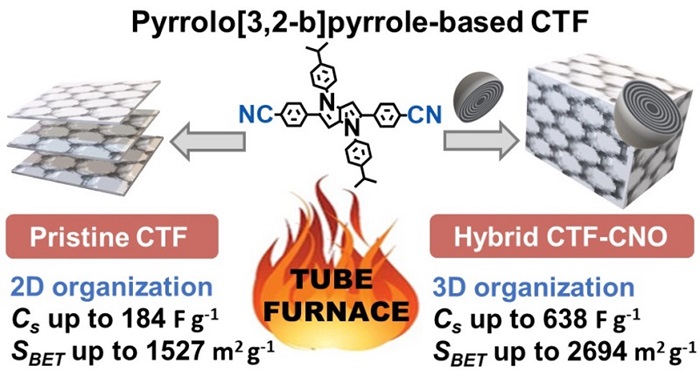
In a recent publication in Scientific Reports (Fig. 2), researchers from the team lead by Professor Marta Płońska-Brzezińska from the Medical University of Białystok show that multilayer fullerenes combined with organic materials (made mainly of carbon and nitrogen) can be used in supercapacitors. Nano-onions are just one of the ingredients of the 'stuffing' that forms the supercapacitor's electrode.
The materials in question are resistant to high temperatures (the existing ones can withstand 600 degrees C), they are excellent semiconductors and accumulate large amounts of charge (Fig. 3). The material is selected to form a porous carbon skeleton. Ions carrying a charge quickly pass through this skeleton to the electrode, and the charge is collected in the material containing carbon nano-onions. The amount of accumulated charge expressed by the specific capacity of the material is 638 F/g. This is one of the highest values for organic materials reported so far in the scientific literature.

Scientists are now working on the best combination of these two types of materials for producing supercapacitor electrodes.
'In the near future, we want to use the possibilities offered by artificial intelligence to design devices with the best possible electrochemical properties. It will help us select materials with great potential that are worth testing,’ says Professor Płońska-Brzezińska. (PAP)
Ludwika Tomala
lt/ bar/ kap/
tr. RL













