
An international team of researchers, including scientists from the University of Warsaw, have described a new method for the synthesis of nucleotides, compounds that are the basic components of DNA and RNA and play a key role in cell signalling and cellular energy processes.
This method is very important from the point of view of the availability of pure, stereochemically modified nucleotides and their use in the development of new therapies. The team's research results were published in Nature Chemistry.
Nucleotides are chemical compounds that are necessary for the functioning of living organisms. They provide energy for cellular processes, help enzymes catalyse specific chemical reactions (they are cofactors), they also take part in intra- and intercellular signalling, they are components of nucleic acids and substrates for their synthesis. Due to these important biological functions, nucleotides also have enormous potential in the context of developing new therapies.
However, since numerous enzymes have the ability to quickly destroy nucleotides, their use in therapeutic processes is complicated. In order to fully use their potential, certain modifications are introduced to change their properties, in particular improve their durability in biological systems', explains Professor Jacek Jemielity from the Centre of New Technologies of the University of Warsaw, co-author of the publication. 'One of the most popular modifications used in this context is the thiophosphate modification, in which a sulphur atom replaces one of the oxygen atoms in the phosphate group,’ he says.
This modification leads to the creation of a completely new chirality centre (also known as a stereogenic centre or asymmetry centre) on the phosphorus atom, which means that it is possible to arrange the substituents in the space around the phosphorus atom in two different ways. This produces two different compounds, called stereoisomers. A common feature of all stereoisomers is that their atoms are connected in the same sequences, but they differ in their spatial arrangement, which may result in different properties.
In order to further study and use the compounds obtained in this way in a therapeutic context, they must be separated into pure stereoisomers, which is often a very difficult and sometimes even impossible process.
Therefore, an international team including scientists from the Faculty of Physics and the Centre of New Technologies of the University of Warsaw and researchers from Scripps Research and the National Institutes of Health (USA) and the University of Bonn (Germany) developed a completely new method - stereocontrolled synthesis of thiophosphate nucleotides.
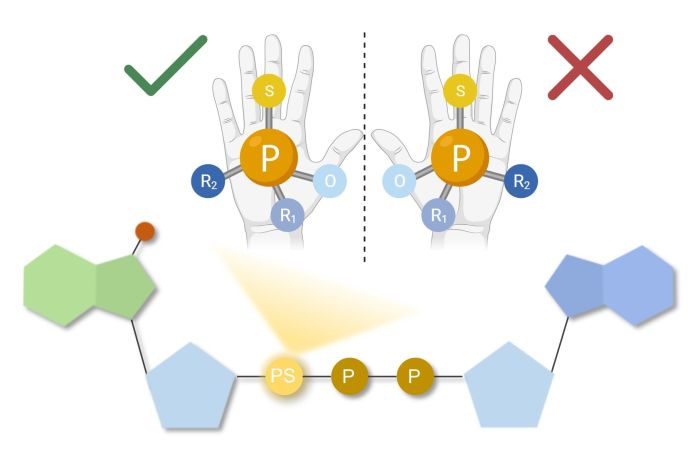
The authors explain that the term 'stereocontrolled' means that the method allows scientists to obtain only one of the two permissible stereoisomers of the nucleotide. The usefulness of the method has been demonstrated on many examples of biologically important nucleotides.
'Among the obtained nucleotides, an important example were new analogues of the 5' end of mRNA, the so-called cap, i.e. reagents used to produce therapeutic mRNA,’ says Dr. Joanna Kowalska from the Faculty of Physics, University of Warsaw. 'The cap structure is extremely important for biological functions and therapeutic applications of messenger RNA (mRNA).’
The researcher emphasises that a team from the University of Warsaw was responsible for investigating the properties of these new compounds.
Thiophosphate cap analogues developed by Polish researchers are already used, for example, in clinical trials of anticancer vaccines based on mRNA technology.
PAP - Science in Poland, Katarzyna Czechowicz
kap/ zan/ kap/
tr. RL













