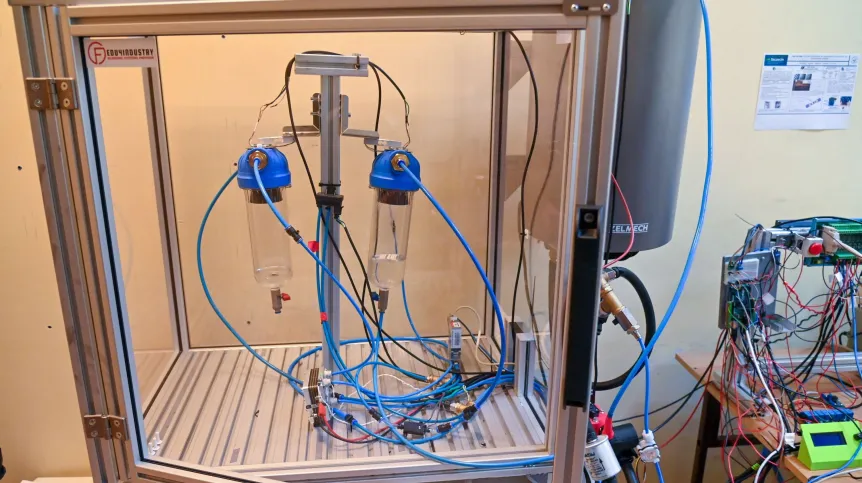
A prototype device called WPUTolyser was created by nanomaterials and mechatronics specialists from the West Pomeranian University of Technology in Szczecin. In this catalyst, which processes water into oxygen and hydrogen, the researchers used iron salts from chemical waste instead of platinum.
Scientists from the Faculty of Chemical Technology and Engineering and the Faculty of Mechanical Engineering and Mechatronics of the West Pomeranian University of Technology in Szczecin have been working on an innovative electrolyser for hydrogen production for several years. The result of their joint research is a prototype device called the WPUTolyser.
'Thanks to the interdisciplinary approach, we designed a durable structure and used waste materials as catalysts. We achieved higher efficiency than commercial solutions based on platinum, iridium or ruthenium', the research initiator, Professor Ewa Mijowska, head of Department of Nanomaterials Physicochemistry at the Faculty of Technology and Chemical Engineering, told PAP.
According to Mijowska, the electrocatalysts availabe on the market contain so-called critical materials, which are expensive because their resources are small. Scientists from all over the world are looking for alternatives. One was found by Szczecin researchers representing two disciplines: materials engineering and mechanical engineering, who analysed post-production waste, including waste from chemical plants in Police near Szczecin.
'Colloquially speaking, we undertook a mission to search through waste produced by various chemical plants in order to find equally or more efficient materials that would generate hydrogen', Mijowska said.
It turned out that after processing, functionalisation and modification, iron-based waste is a very efficient electrocatalyst. Mijowska emphasised that the production of hydrogen and oxygen from water (splitting a water molecule under the influence of an electric current) is a process that 'has remained a mystery to scientists for 200 years'.
'It only took us a few years to be where we are today', said the head of the Szczecin research.
She assured that WPUTolyser was ready for implementation work. This was possible thanks to the cooperation of Mijowska's team with specialists from the Hydrogen Technologies Laboratory at the West Pomeranian University of Technology.
'We have created technical support for the transition from the microelectrolyser scale to a laboratory prototype. We can use it to make an electrolyser fully suitable for commercial applications', says Professor Piotr Pawełko, head of the Mechatronics Department at the West Pomeranian University of Technology.
The technical support included building equipment, a water supply system, temperature monitoring, and measuring the amounts of produced oxygen and hydrogen. Pawełko emphasises that most of the technical solutions were the work of Łukasz Mozga, PhD. The aim of the research was primarily to increase the electrolyser's efficiency.
'Not only through the ordinary action of current on water and the creation of hydrogen and oxygen, but also through physical phenomena, and more precisely through fluid mechanics phenomena', Pawełko explains.
Computer analysis of fluid mechanics enables the optimal generation of flow through the electrolyzer; the West Pomeranian University of Technology scientists are pioneers in this area of research. 'We are slowly starting to model it and the efficiency is being increased', Pawełko assures.
Another major technical challenge was to apply an iron sulphate catalyst to the so-called PEM (Proton Exchange Membrane). It turned out that it was difficult to 'stick' it to a plastic plate. However, when you act on it with a laser, it becomes 'more rough, more willing to react'. 'With a pressure of 200 bar, gas, nitrogen, we will press it into the membrane', Pawełko describes this process.
In order to develop a technology for making a membrane that would let hydrogen through but stop oxygen, experiments were carried out in the Faculty of Mechanical Engineering and Mechatronics laboratory, in a special chamber at pressures of up to 400 bar.
WPUTolyser has achieved efficiency similar to commercial electrolysers. At the appropriate process temperature, the prototype device produces 0.6 litres of hydrogen per minute, when powered from a regular power grid, up to 3 kW. Scientists point out that the more hydrogen you want to produce, the more electricity you have to use. That is why they emphasise that the installation they have developed can be configured with renewable energy sources, adapted to a local power source.
'It is easy to combine our electrolyser with renewable energy sources, such as photovoltaics or wind turbines, so that this electrolyser is 100% green. So that it does not generate any harmful gases', summarises Klaudia Maślana, PhD from the Department of Physical Chemistry and Nanomaterials.
Tomasz Maciejewski (PAP)
PAP - Science in Poland
tma/ bar/ lm/













